Thermochemistry Intro
Uploaded by
Filip MarkusThermochemistry Intro
Uploaded by
Filip MarkusThermochemistry
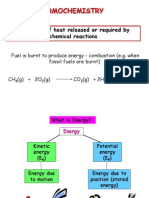
is the study of energy changes that occur
during chemical reactions and changes in
state.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
The law of conservation of energy
• states that in any chemical or physical process, energy is neither created
nor destroyed
• the energy of the universe remains unchanged
•If the energy of the system increases during
that process, the energy of the surroundings
must decrease by the same amount.
•If the energy of the system decreases during
that process, the energy of the surroundings
must increase by the same amount.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Recognizing Endothermic and Exothermic
Processes
On a sunny winter day, the snow on a rooftop
begins to melt. As the melted water drips from
the roof, it refreezes into icicles. Describe the
direction of heat flow as the water freezes.
Is this process endothermic or exothermic?
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
2 Solve Apply concepts to this situation.
First identify the system and the surroundings.
System: water
Surroundings: air
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
2 Solve Apply concepts to this situation.
Determine the direction of heat flow.
• In order for water to freeze, its temperature
must decrease.
• Heat flows out of the water and into
the air.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
2 Solve Apply concepts to this situation.
Determine if the process is endothermic or exothermic.
• Heat is released from the system to the
surroundings.
• The process is exothermic.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Athletes often use instant cold packs to soothe
injuries. Many of these packs use the dissociation of
ammonium nitrate in water to create a cold-feeling
compress.
Is this reaction endothermic or exothermic? Why?
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Athletes often use instant cold packs to soothe
injuries. Many of these packs use the dissociation of
ammonium nitrate in water to create a cold-feeling
compress.
Is this reaction endothermic or exothermic? Why?
The instant cold pack feels cold because it removes heat from
its surroundings. Therefore, the dissociation of ammonium
nitrate in water is endothermic. The system (the cold pack)
gains heat as the surroundings lose heat.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Recognizing Endothermic and Exothermic
Processes
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Recognizing Endothermic and Exothermic
Processes
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Recognizing Endothermic and Exothermic
Processes
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Recognizing Endothermic and Exothermic
Processes
exothermic endothermic
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
The specific heat capacity, or simply the specific
heat, is the amount of heat it takes to raise the
temperature of 1 g of the substance 1°C.
Specific Heats of Some Common Substances
• Water has a
very high
specific heat
compared with
the other
Specific heat
substances. Substance
J/(g·°C) cal/(g·°C)
• Metals Liquid water 4.18 1.00
generally have Ethanol 2.4 0.58
low specific Ice 2.1 0.50
heats. Steam 1.9 0.45
Chloroform
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Heat Capacity and
Specific Heat of Water
Specific Heat
Just as it takes a lot of heat to raise the temperature
of water, water also releases a lot of heat as it cools.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Heat Capacity and
Specific Heat
Specific Heat of Water
Water in lakes and oceans absorbs heat from the air
on hot days and releases it back
into the air on cool days.
• This property of water is
responsible for moderate climates
in coastal areas.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Heat Capacity and
Specific Heat of Water Specific Heat
When a freshly baked apple pie comes out of the oven,
both the filling and the crust are at the same temperature.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Heat Capacity and
Specific Heat of Water Specific Heat
When a freshly baked apple pie comes out of the oven,
both the filling and the crust are at the same temperature.
• The filling, which is mostly water, has a
higher specific heat than the crust.
• In order to cool down, the filling must
give off a lot of heat.
• This release of heat is why you have to
be careful not to burn your tongue when
eating hot apple pie.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Heat will flow from the lava to the surroundings until the
lava and surroundings are at the same temperature. Air has
a smaller specific heat than water.
Where would lava cool more quickly in water or in air?
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Heat will flow from the lava to the surroundings until the
lava and surroundings are at the same temperature. Air has
a smaller specific heat than water.
Where would lava cool more quickly in water or in air?
Water requires more energy to raise
its temperature than air.
Therefore, lava in contact with water
loses more heat energy than lava in
contact with air, allowing it to cool
more quickly.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Heat Capacity and
Specific Heat
Calculating Specific Heat
To calculate the specific heat (C) of a substance, you
divide the heat input by the mass of the substance
times the temperature change.
q heat (J or cal)
C= =
o
m ΔT mass (g) change in temperature ( C)
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Calculating the Specific Heat of a Substance
The temperature of a 95.4-g piece of copper increases from
25.0°C to 48.0°C when the copper absorbs 849 J of heat.
What is the specific heat of copper?
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
Evaluate Does the result make sense?
3
• Remember that liquid water has a specific heat of
4.18 J/(g·°C).
• Metals have specific heats lower than water.
• Thus, the calculated value of 0.387 J/(g·°C) seems
reasonable.
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
The specific heat of ethanol is 2.4 J/(g·°C). A sample of
ethanol absorbs 676 J of heat, and the temperature rises
from 22°C to 64°C. What is the mass of ethanol in the
sample?
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
The specific heat of ethanol is 2.4 J/(g·°C). A sample of
ethanol absorbs 676 J of heat, and the temperature
rises from 22°C to 64°C. What is the mass of ethanol in
the sample?
q q
C= m ΔT m= C ΔT
676 J
m= = 6.7 g ethanol
2.4 J/(g·°C) (64°C – 22°C)
COPYRIGHT © BY SAVVAS LEARNING COMPANY LLC. ALL RIGHTS
RESERVED.
You might also like
- Exothermic and Endothermic Reactions Board Works100% (1)Exothermic and Endothermic Reactions Board Works15 pages
- Larry D. Buban, PH.D Science Education - Physics Cas - WvsuNo ratings yetLarry D. Buban, PH.D Science Education - Physics Cas - Wvsu49 pages
- Chemistry For Engineers - 1 Energy - Topic 02 - Sensible Heat-Heat Capacity-CalorimetryNo ratings yetChemistry For Engineers - 1 Energy - Topic 02 - Sensible Heat-Heat Capacity-Calorimetry7 pages
- C11 Thermal Properties of Matter CompressedNo ratings yetC11 Thermal Properties of Matter Compressed10 pages
- Heat and Temperature: The Heat, As Glen Frey Says, Is OnNo ratings yetHeat and Temperature: The Heat, As Glen Frey Says, Is On27 pages
- Heat Is A Form of Energy by Absorbing Heat A Body Becomes HotterNo ratings yetHeat Is A Form of Energy by Absorbing Heat A Body Becomes Hotter6 pages
- 1A03-2024-08-Thermochemistry-skeleton notesNo ratings yet1A03-2024-08-Thermochemistry-skeleton notes55 pages
- Chapter 17 Thermochemistry PPT Marquart GOODNo ratings yetChapter 17 Thermochemistry PPT Marquart GOOD82 pages
- Thermochemistry: The Study of Heat Released or Required by Chemical ReactionsNo ratings yetThermochemistry: The Study of Heat Released or Required by Chemical Reactions31 pages
- 3.3 Relationship Between Thermal Energy, Specific Heat Capacity, Mass and Temperature ChangeNo ratings yet3.3 Relationship Between Thermal Energy, Specific Heat Capacity, Mass and Temperature Change11 pages
- Endothermic and Exothermic Reactions MSNo ratings yetEndothermic and Exothermic Reactions MS14 pages
- Daily Lesson LOG: Monday Tuesday Wednesday Thursday FridayNo ratings yetDaily Lesson LOG: Monday Tuesday Wednesday Thursday Friday4 pages
- Class - 10th Chemistry Chapter 1 Chemical Equations PDF50% (2)Class - 10th Chemistry Chapter 1 Chemical Equations PDF248 pages
- Science 8 Chapter 8 Chemical Reaction 8.1-8.250% (2)Science 8 Chapter 8 Chemical Reaction 8.1-8.217 pages
- NCERT Solutions For Class 10th Science - Chapter 1 Chemical Reactions and EquationsNo ratings yetNCERT Solutions For Class 10th Science - Chapter 1 Chemical Reactions and Equations12 pages
- Cambridge Lower Secondary Checkpoint: Science 1113/0269% (13)Cambridge Lower Secondary Checkpoint: Science 1113/0214 pages
- Thermo Chemistry: The Study of Changes in Heat Energy During Chemical Reaction100% (1)Thermo Chemistry: The Study of Changes in Heat Energy During Chemical Reaction20 pages
- Thermochemical Energy Storage PresentationNo ratings yetThermochemical Energy Storage Presentation10 pages
- Exercise 1 Thermodynamics: A Review: ObjectivesNo ratings yetExercise 1 Thermodynamics: A Review: Objectives5 pages
- Larry D. Buban, PH.D Science Education - Physics Cas - WvsuLarry D. Buban, PH.D Science Education - Physics Cas - Wvsu
- Chemistry For Engineers - 1 Energy - Topic 02 - Sensible Heat-Heat Capacity-CalorimetryChemistry For Engineers - 1 Energy - Topic 02 - Sensible Heat-Heat Capacity-Calorimetry
- Heat and Temperature: The Heat, As Glen Frey Says, Is OnHeat and Temperature: The Heat, As Glen Frey Says, Is On
- Heat Is A Form of Energy by Absorbing Heat A Body Becomes HotterHeat Is A Form of Energy by Absorbing Heat A Body Becomes Hotter
- Thermochemistry: The Study of Heat Released or Required by Chemical ReactionsThermochemistry: The Study of Heat Released or Required by Chemical Reactions
- 3.3 Relationship Between Thermal Energy, Specific Heat Capacity, Mass and Temperature Change3.3 Relationship Between Thermal Energy, Specific Heat Capacity, Mass and Temperature Change
- On Specific Heat, Pyrolysis, Transponders, Et AliiFrom EverandOn Specific Heat, Pyrolysis, Transponders, Et Alii
- Daily Lesson LOG: Monday Tuesday Wednesday Thursday FridayDaily Lesson LOG: Monday Tuesday Wednesday Thursday Friday
- Class - 10th Chemistry Chapter 1 Chemical Equations PDFClass - 10th Chemistry Chapter 1 Chemical Equations PDF
- NCERT Solutions For Class 10th Science - Chapter 1 Chemical Reactions and EquationsNCERT Solutions For Class 10th Science - Chapter 1 Chemical Reactions and Equations
- Cambridge Lower Secondary Checkpoint: Science 1113/02Cambridge Lower Secondary Checkpoint: Science 1113/02
- Thermo Chemistry: The Study of Changes in Heat Energy During Chemical ReactionThermo Chemistry: The Study of Changes in Heat Energy During Chemical Reaction